EKSPO FARMA LTD established as a medical health care products sales company stand in 2007. Its first international activity was attending the FİME 2007 Medical Trade Fair – Miami in the USA .
After many years internal and external trade experinces, our goal is producing equivalent high technology medical devices and health care products which have been importing in Turkey for many years. Increasing sales, expanding our business and customer satifaction are our objectives.
Ekspo Farma is the World’s First Integrated Urology Company to be Certified by the European Union’s Medical Device Regulation.
The MDR certification replaced the former EU Medical Device Directive (MDD) on 26th May 2021. It includes a more robust legislative framework that enforces a higher standard of public health and patient safety.






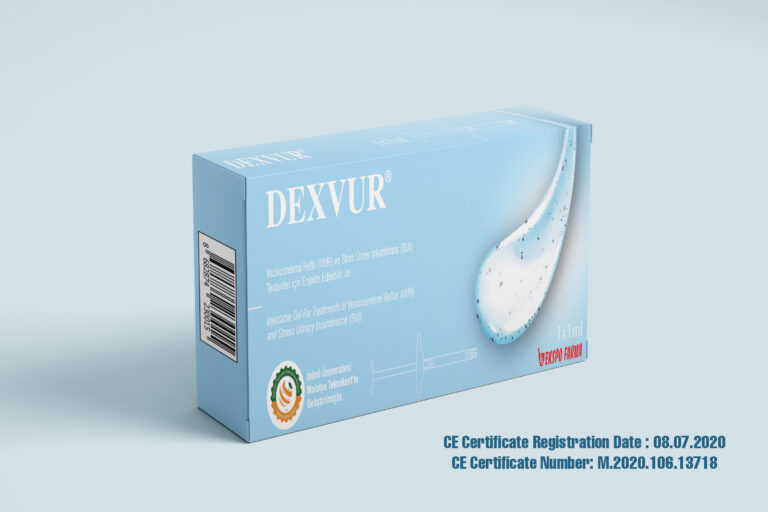
 In the case of the development of our medical device called ‘’ Injectable Gel for treatments of Vesicoureteral Reflux (VUR) and Stress Urinary Incontinence (SUI)’’, we have been proceeding our facilities under ISO 13485 standarts for Design and Development and Manufacturing Management of the medical device and CE certificated it on July 2020. For the development of the medical device, we have permission from Turkish Ministry of Health Ethics Committee Decision for performing clinical trials and completed clinical trials.
In the case of the development of our medical device called ‘’ Injectable Gel for treatments of Vesicoureteral Reflux (VUR) and Stress Urinary Incontinence (SUI)’’, we have been proceeding our facilities under ISO 13485 standarts for Design and Development and Manufacturing Management of the medical device and CE certificated it on July 2020. For the development of the medical device, we have permission from Turkish Ministry of Health Ethics Committee Decision for performing clinical trials and completed clinical trials.